The alkynes ko hydrocarbons na acetylenic su ne hydrocarbons wanda ke nuna kasancewar haɗin carbon-carbon sau uku. Alkynes marasa cyclic suna amsa tsarin kwayoyin CnH2n-2. Suna da matakin kafa har ma ya fi na alkenes.
Daga cikin kaddarorin sunadarai na alkynes yana nuna gaskiyar cewa su ne ƙananan polarity mahadi, saboda haka insoluble cikin ruwa, amma mai narkewa a cikin abubuwan narkar da ƙwayoyin cuta na yau da kullun kamar ether, benzene, ko carbon tetrachloride.
Thetafasasshen wuraren narkewa na alkynes sun yi kama da na alkanes ko alkenes na daidai adadin carbon. A gefe guda kuma, ana iya ganin tasirin tasirin waɗannan madaidaitan na jiki, wanda ke da adadin carbons da kasancewar rassa a cikin sarkar (wanda ke canzawa a cikin alkibla ɗaya), ya fi dacewa.
Mafi alkyne shine acetylene, suna bin propylene (ko tip) da kuma butino, wanda zai iya zama 1-butyne (haɗin sau uku a ƙarshen ƙwayar) ko 2-butyne (haɗin sau uku a tsakiyar kwayar). Wadannan ukun sune gas; wadanda suke da mafi yawan adadin sinadarin carbon ruwa ko m.
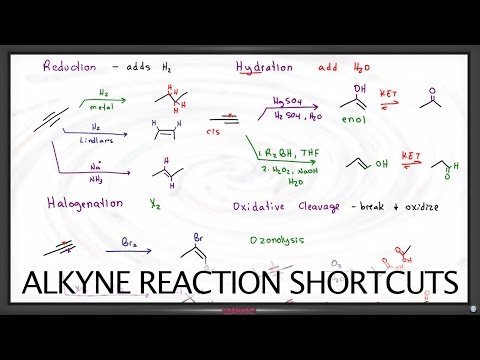
Hakazalika da alkenes, abubuwan haɗin gwiwa guda uku waɗanda ke nuna alkynes suna ba su babban sinadarin reactivity Waɗannan abubuwan kuma suna sa su zama masu saurin kamuwa da ƙarin halayen (hydrogen, halogens, ruwa, da sauransu) da sauransu. Koyaya, shaidu guda uku waɗanda ke haɗa atom ɗin carbon ɗaya tare da wani ba daidai bane: ɗayansu (wanda ake kira sigma bond) yana da ƙarfi kuma yana aiki azaman babban alhakin ƙungiyar. Haɗin da ke haifar zai iya kasancewa yana da shaidu biyu ko kuma guda ɗaya kawai.
Ƙungiyoyin da ke da haɗin sau uku a ƙarshen sarkar ana kiran su m alkynes; waɗannan mahadi ana rarrabe su da alamar acidity. A zahiri, alkynes masu ƙarewa sune mafi sauƙin hydrocarbons mai sauƙin acidic. Tsawon atoms carbon a cikin haɗin shine 1.20 amstrongs (har ma ya fi guntu na alkenes ─1.34 amstrongs─- da na alkanes ─1.54 amstrongs─). Singleaukaka guda biyu, sau biyu da sau uku na carbon-carbon zasu iya zama tare a cikin ƙwayoyin guda ɗaya. Lokacin da wannan ya faru, ana kiran hydrocarbon a matsayin alkyne kuma an nuna matsayin haɗin biyun tare da ƙarshen "ene", saka shi a inda ya dace
Misalan alkynes an jera su a ƙasa:
- ethyne (acetylene)
- tip
- 2- pentin
- 2-butyne
- 1-butyne
- Oktoba 3
- 2-nonino
- Methyl acetylene
- Ya ƙunshi ethyl acetylene
- 1-ene-4-hexyne
- Propyl acetylene
- Terbutyl acetylene
- 6,6-diethyl-4-noniino
- 5,6-dimethyl-3-heptin
- 3,3-diethyl-3,5-nonadiino
- cyclobutine
- 3-ethyl -5-ethynylhepta-1,6-diino
- 5-methyl-2-hexyne
- 3,5,7-decatriino
- 6-methyl-2,4-heptadiino