Wadatacce
A cikin ilmin sunadarai, akwai maganar a Gishiri lokacin da muke magana mahadi da ake samu lokacin da acid yana da sinadarin hydrogen ɗinsa wanda aka maye gurbinsa da tsattsauran ra'ayi, wanda a cikin takamaiman yanayin salts na acid, sune iri iri (cations). A cikin haka an bambanta su daga gishiri mai tsaka tsaki ko gishiri na binary.
Gishiri galibi ana samun su ta hanyar amsawa tsakanin acid da hydroxide (tushe). A cikin waɗannan halayen, galibi tushe yana rasa ƙungiyoyin hydroxyl (-OH) da acid hydrogen atom (H), suna yin gishiri mai tsaka tsaki; amma idan acid ɗin da ake tambaya ya adana ɗayan atom ɗin hydrogen ɗin sa, yana canza cajin wutar lantarki, za mu sami gishiri acid ko gishiri hydrogenated.
Don haka, alal misali, ana samun lithium bicarbonate daga lithium hydroxide da carbonic acid:
LiOH + H.2CO3 = Li (HCO3) + H da2KO
Halin, kamar yadda za a gani, shima yana jefa ruwa azaman samfuri.
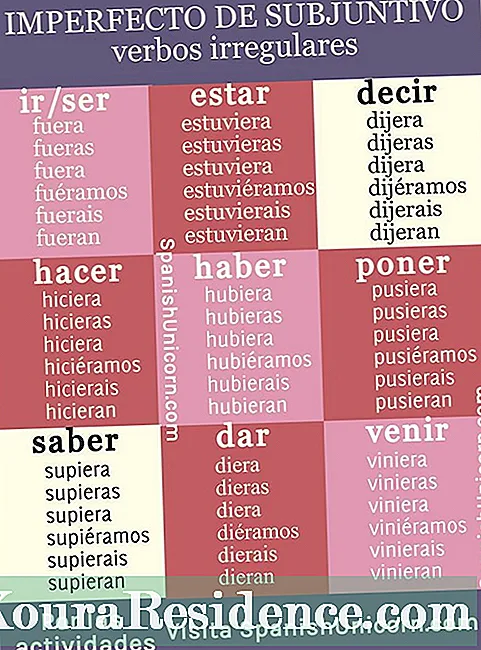
Nomenclature na salts acid
Dangane da nomenclature na aiki, don saltsin acid yakamata a yi amfani da hanyar gargajiya ta sanya sunan gishiri mai tsaka tsaki daga kari -ate ko -ite, amma ya riga da prefix wanda ke nuna adadin ƙwayoyin hydrogen da aka musanya a kan molecule. Don haka, alal misali, lithium bicarbonate (LiHCO3) zai sami atomin hydrogen guda biyu (bi = biyu).
A gefe guda kuma, bisa ga tsarin nomenclature, kalmar hydrogen zuwa sunan talakawa na gishiri da aka samu, game da prefixes da ke magana akan maye gurbin atom din hydrogen. Don haka, lithium hydrogen carbonate ko lithium hydrogen carbonate zai zama hanyoyi na sanya sunan wannan lithium bicarbonate (LiHCO)3).
Misalan ruwan gishiri
- Sodium bicarbonate (NaHCO3). Har ila yau ana kiranta sodium hydrogen carbonate (IV), farin farin ƙarfe ne, mai narkewa cikin ruwa, wanda za'a iya samu a yanayi a cikin yanayin ma'adinai ko ana iya samarwa a cikin dakin gwaje -gwaje. Yana daya daga cikin gishiri mafi yawan acidic da aka sani kuma ana amfani dashi sosai a cikin kayan zaki, magunguna ko yin yogurt.
- Lithium bicarbonate (LiHCO3). Anyi amfani da wannan gishirin acid azaman wakilin kamawa na CO2 a cikin yanayi inda irin wannan gas ɗin ba a so, kamar a Arewacin Amurka "Apollo" ayyukan sararin samaniya.
- Potassium dihydrogen phosphate (KH2PO4). M crystalline, m, wari, mai narkewa cikin ruwa, yadu ana amfani dashi a masana'antu daban -daban kamar yisti na abinci, wakilin chelating, mai ba da abinci mai gina jiki da mataimaki a cikin hanyoyin dafawa.
- Sodium bisulfate (NaHSO4). Gishirin acid wanda aka samu ta hanyar tsaka -tsakin sulfuric acid, wanda ake amfani da shi sosai a masana'antar sarrafa baƙin ƙarfe, samfuran tsaftacewa kuma kodayake yana da guba sosai ga wasu echinoderms, ana amfani dashi azaman ƙari a cikin abincin dabbobi da ƙera kayan ado.
- Sodium hydrogen sulfide (NaHS). Haɗari mai haɗari na kulawa mai daɗi, tunda yana da lalata sosai da guba. Yana iya haifar da kone -konen fata da lalacewar ido, saboda shi ma yana iya konewa.
- Calcium hydrogen phosphate (CaHPO4). Anyi amfani dashi azaman ƙarin abinci a cikin hatsi da abincin dabbobi, yana da ƙarfi mai narkewa a cikin ruwa amma yana iya crystallizing lokacin da aka shayar da shi ta hanyar cinye ƙwayoyin ruwa guda biyu.
- Ammonium hydrogen carbonate ([NH4] HCO3). An san shi da ammonium bicarbonate kuma ana amfani dashi a masana'antar abinci azaman yisti na sunadarai, kodayake yana da raunin tarko ammoniya, yana ba abinci mummunan dandano idan aka yi amfani da shi fiye da kima. Hakanan ana amfani dashi a cikin masu kashe gobarar wuta, yin launi, kuma azaman mai faɗaɗawar roba.
- Barium bicarbonate (Ba [HCO3]2). Gishirin Acidic wanda idan mai zafi zai iya juyar da aikin samar da shi kuma yana da tsayayye sosai sai a cikin mafita. Ana amfani dashi sosai a masana'antar yumbu.
- Sodium bisulfite (NaHSO3). Wannan gishirin ba shi da ƙarfi sosai kuma a gaban iskar oxygen yana shiga cikin sodium sulfate, wanda shine dalilin da yasa ake amfani dashi a masana'antar abinci azaman mai kiyaye abinci da bushewa. Babban wakili ne mai raguwa kuma galibi mutum yana amfani da shi, kuma ana amfani dashi wajen gyara launuka.
- Calcium citrate (Ca3[C6H5KO7]2). Wanda aka fi sani da gishiri mai ɗaci, ana amfani dashi azaman kayan abinci kuma azaman ƙarin abinci mai gina jiki lokacin da aka haɗa shi da amino acid lysine. Fari ne, mara ƙamshi, ƙura mai ƙyalli.
- Monocalcium phosphate(Ka [H2PO4]2). M m launi wanda aka samu daga dauki na alli hydroxide da phosphoric acid, Ana amfani dashi sosai azaman wakili mai yisti ko a matsayin taki a aikin noma.
- Dicalcium phosphate (CaHPO4). Har ila yau aka sani da alli monohydrogen phosphate, yana da uku daban -daban crystalline siffofin cewa Ana amfani da su azaman ƙari a cikin abinci kuma yana nan a cikin ɗan goge baki. Bugu da ƙari, an ƙirƙira shi ta halitta a cikin duwatsun koda da abin da ake kira “dutse”.
- Monomagnesium phosphate (MgH4P2KO8). Anyi amfani dashi azaman mai haɓaka acidic, mai gyara acidity ko wakili a cikin lura da gari, ba shi da wari, farin gishiri mai ƙyalli, wanda ɗan narke cikin ruwa kuma ana amfani da shi wajen adana abinci.
- Diacetate sodium (NaH [C2H3KO2]2). Ana amfani da wannan gishirin azaman wakili na ɗanɗano da mai kiyayewa don abinci, yana hana ko jinkirta bayyanar fungi da microbacteria, duka a cikin kayan da aka cika kamar kayayyakin nama da masana'antar gari.
- Calic bicarbonate (Ca [HCO3]2). Gishirin Hydrogenated wanda ya samo asali daga sinadarin carbonate na calcium, wanda ke cikin ma'adanai kamar limestone, marmara da sauran su. Wannan halayen yana nuna kasancewar ruwa da CO2, don haka yana iya faruwa kwatsam a cikin kogo da kogon da ke da alli.
- Rubidium acid fluoride (RbHF). Ana samun wannan gishirin daga halayen hydrofluoric acid (hydrogen da fluorine X) da Rubidium, ƙarfe alkali. Sakamakon sakamako ne mai guba da gurɓataccen abu wanda dole ne a kula da shi da hankali..
- Monoammonium phosphate ([NH4] Da H.2PO4). Gishiri mai narkewa na ruwa wanda aka samar ta hanyar ammonia da phosphoric acid, a ko'ina Ana amfani dashi azaman taki tunda yana samar da ƙasa tare da sinadarin nitrogen da phosphorus waɗanda ake buƙata don haɓaka shuka. Hakanan yana cikin foda na ABC a cikin masu kashe gobara.
- Zinc hydrogen orthoborate(Zn [HBO3]). Gishiri da ake amfani da shi azaman maganin kashe ƙwari da azaman ƙari a cikin samar da yumɓu.
- Monosodium phosphate (NaH2PO4). An yi amfani da mafi yawa a dakunan gwaje -gwaje, kamar “buffer”Ko kuma mafita mai ɓoyewa, wanda ke hana canje -canje kwatsam a cikin pH na mafita.
- Potassium hydrogen phthalate (KHP). Har ila yau ana kiranta potassium acid phthalate, gishiri ne mai ƙarfi da tsayayye a cikin iska ta yau da kullun, don haka galibi ana amfani dashi azaman ma'aunin farko a ma'aunin ma'aunai pH. Hakanan yana da amfani azaman wakilin buffering a ciki halayen sunadarai.
Yana iya ba ku:
- Misalan Gishirin Ma'adinai da aikinsu
- Misalan Gishirin Neutral
- Misalan gishirin Oxisales